In 2021, the media was flooded with issues related to vaccine equity and vaccine manufacturing in Africa. Many organisations globally raised these issues and proposed various ways to address this inequity, both to meet the needs of the continent during the Covid-19 pandemic and to create a state of self-sufficiency and competitiveness through local manufacturing.
But what does this mean in practice?
What we know is that pharmaceuticals were ranked the fourth-highest category in terms of value of imports to sub-Saharan Africa in 2018 by the World Integrated Trade Solution, amounting to approximately 70% — 90% of the total sub-Saharan Africa market for medicines. In sharp contrast, according to Unctad, the value of exports of pharmaceuticals in the same year was less than 10% of the value of pharmaceutical imports.
When looking at the vaccine manufacturing landscape in Africa, the situation is even more dire than it is for pharmaceuticals: currently, vaccine production is estimated to meet around 1% of the domestic need of the continent for routine immunisation — this does not include needs for pandemic and outbreak control. Yet, Africa makes up about 25% of global vaccine demand.
According to the WHO’s 2019 Global Vaccine Market Report, global vaccine purchase data released by the WHO indicates potential supply security challenges in two key areas:
1) where there are too few suppliers of individual vaccines or vaccine components; and
2) where countries are limiting their available choices by relying predominantly on supply from certain manufacturers or manufacturer groups.
The global procurement and supply chains of vaccines are currently dominated by a limited number of manufacturers in a small number of countries and need to be expanded and diversified to improve health security.
In a recent article published on Devex, WHO Director-General Dr Tedros Adhanom Ghebreyesus is quoted as saying that “Protectionist approaches to manufacturing and trade will only aid the spread of the virus, we must act globally and ensure that tools such as vaccines can be produced worldwide if we are to end the acute stage of this pandemic.” Ghebreyesus points out that: “No other event like the Covid-19 pandemic has shown that reliance on a few companies to supply global public goods is limiting, and dangerous.” (Read more here)
In almost all policy discussions, there has been broad agreement that African countries should develop new and ramp up existing local vaccine manufacturing capabilities through public-private partnerships, investing in manufacturing infrastructure, technology transfer, etc.
However, as with many policy conversations that have come before, these discussions and debates have stopped short of the practical implementation aspects that will be required to create long-term sustainability at a local level; what capabilities would be required to achieve this? What opportunities and natural advantages do we have that can be leveraged to enhance efficiencies in the value chain?
Answering these questions is going to be critical if we are going to create lasting resilience in our healthcare systems and if we are to successfully break the cycles of external dependency that plague us today.
Some would argue that African countries should not even pursue developing drug substance (DS) manufacturing capabilities at all due to the level of complexity and the late stage that we would be entering these markets.
Partnership for African vaccine manufacturing
In response to the critical and urgent need for vaccines, in April 2021 heads of state from across the continent met to make a collective commitment to having at least 60% of Africa’s consumption of vaccines for routine immunisation being manufactured on the continent by 2040 (Read: African Union and Africa CDC launches Partnerships for African Vaccine Manufacturing (PAVM), framework to achieve it and signs 2 MoUs). This important task was delegated to the Africa Centres for Disease Control and Prevention (Africa CDC) which facilitated the inauguration of the Partnership for African Vaccine Manufacturing (PAVM) in April 2021 together with the establishment of several technical task teams with experts from Africa.
Africa CDC’s PAVM initiative has led to at least nine countries on the continent starting (or planning) multiple initiatives towards expanding domestic manufacturing capacity on the continent, starting with Covid-19 vaccines (Algeria, Egypt, Ghana, Morocco, Nigeria, Rwanda, Senegal, South Africa, Uganda).
Some of these countries have taken the bold step of leapfrogging to manufacture using newer platform technologies like the mRNA platform vaccine. Recently, the first Covid-19 messenger RNA (mRNA) vaccine technology transfer hub on the African continent was also set up in South Africa. Other African entrepreneurs are looking at developing novel vaccines using “virus-like particle” technology.
To date, most of the vaccine manufacturing initiatives and partnerships in Africa have focused on the latter part of the value chain, ie “fill & finish”. This focus is very much needed and a necessary step towards developing local African vaccine manufacturing capacity — however, this does not alleviate the external dependency since the DS or active ingredient for the vaccines still need to be imported from the original manufacturer.
Access to DS remains a challenge due to intellectual property arrangements as well as limited capacity to manufacture DS in Africa. However, Moderna recently announced the first mRNA manufacturing facility to be established in Kenya, although the practical arrangements and the benefits to the local market remain unclear. The facility will focus on manufacturing vaccine drug substances, with potential plans to expand its capacity to also include the filling of vaccine vials “as early as 2023, subject to demand”.
It also announced a new initiative “that will offer researchers use of Moderna’s mRNA technology to explore new vaccines against emerging or neglected infectious disease”, which will allow researchers globally access to the company’s “preclinical manufacturing capabilities and research and development expertise.”(Read: The looming Covid-19 treatment equity gap)
Even though the pandemic has created a high level of momentum in the rush to develop local vaccine manufacturing capabilities, there is also a fair amount of scepticism around African countries’ capabilities to implement and maintain this level of manufacturing infrastructure. There is also the risk of duplication of effort and capabilities across countries which may become problematic when considering access to markets.
Due to the urgency of the pandemic and the need to optimise speed and quality, there has been limited opportunity to source any of the production input materials required for the manufacturing of vaccines locally from African sources due to stringent regulatory requirements. Changes in the source of production inputs or manufacturing process methods would require regulatory approval and, if significant, could require new clinical trials due to the potential clinical impact on patients.
 Vaccine production and deployment process.
Vaccine production and deployment process.
Adapted from Plotkin, et al, The complexity and cost of vaccine manufacturing – an overview.)
Vaccines are often produced using raw materials produced by biological production processes (eg yeast extract, natural or recombinant enzymes). These materials add inherent biological variability to the manufacturing or analytical processes. Due to their specialised nature, these raw materials may be limited in supply, and subject to shortages or process changes as suppliers change methods to increase productivity or improve their bottom line.
Equipment, production inputs and skilled labour that are not readily available in low-resource countries would need to be imported, potentially maintained remotely or replaced, for years, driving up manufacturing costs. Countries seeking to augment or localise vaccine supply will need to invest heavily in facilities, equipment, development of local skilled labour forces and ongoing quality management with a long time horizon and they will have to carefully weigh the systemic risks and inherent challenges in high-quality vaccine manufacture over the long term.
Other workstreams within the PAVM framework (such as pathways for regulatory strengthening, infrastructure development and technology and intellectual property) require the support of the Africa Continental Free Trade Area (AfCFTA) Secretariat to establish optimised value chains for sustainable manufacturing of vaccines and other pharmaceuticals on the African Continent.
The AfCFTA, which aspires to create one single market in Africa, allows us opportunities for cooperation and collaboration that were not available previously. The AfCFTA is an important enabler of vaccine manufacturing in Africa, however, it is not an end in itself but rather presents us with an opportunity for economic transformation and industrialisation through the building of productive capacity, promotion of economic diversification, structural transformation, and technological development.
There are also the non-manufacturing countries in Africa to think about — what role could they play in offering either production inputs or even services into healthcare value chains to further localise and create efficiencies?
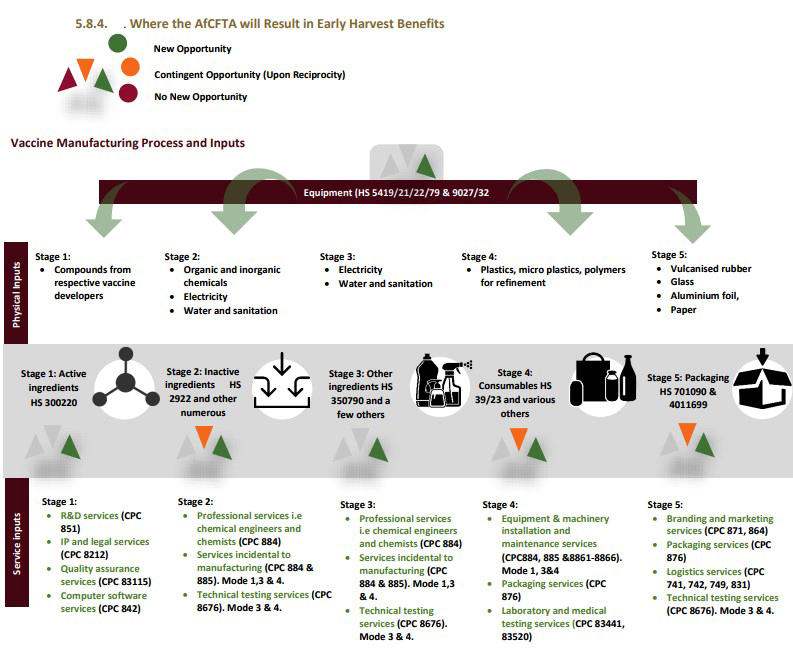
There are at least 150-200 production inputs that are required in the vaccine production process. Many of these production inputs are common across different vaccine platforms and provide potential opportunities for localisation. The Futures report, 2021, commissioned by the UNDP and the AfCFTA Secretariat also identified various opportunities across the vaccine value chain, both in terms of physical and service inputs, that could be potentially localised.
All of these opportunities would need to be further investigated and could potentially provide opportunities to those non-manufacturing countries to become service or production input providers in the vaccine value chain — this is how we could begin to create regional value chains, self-sufficiency and healthcare resiliency which are some of the continent’s aspirations.
Advances in technology can be leveraged to contribute toward efficiencies in the value chain and enhance competitiveness through approaches such as continuous manufacturing, environmental sustainability, artificial intelligence, quality by design and so on.
As healthcare innovation progresses and public health is confronted with multiple unexpected shocks, as demonstrated by the current pandemic, there is a need for African countries to focus on agile approaches to manufacturing processes and design them with a view to ensuring that they are adaptable and fit for purpose for multiple needs in the future while minimising the potential for redundancy.DM/MC
On 13 April 2022, the Nelson Mandela School of Public Governance will host a webinar on active ingredient manufacturing for vaccines. The webinar is the second in a series of roundtable discussions that aim to make actionable policy recommendations to develop regional value chains in the healthcare and pharmaceutical sector across Africa through leveraging opportunities presented by the implementation of the African Continental Free Trade Agreement (AfCFTA). Register to attend: https://forms.gle/q9PSs4oqM8fMJkFV8
Kirti Narsai has 24 years’ experience in the healthcare industry. She was a member of the African Regional Business Network of the WEF Africa. She was appointed as an expert panel member advising the SA government on developing a Health Master Plan and to the Market Design & Demand Intelligence workstream of the Partnership for African Vaccine Manufacturing (PAVM). She runs her own consultancy and also holds a part-time position as Principal Researcher at the Nelson Mandela School of Public Governance leading research on pharmaceutical value chains in the context of the AfCFTA. Previously she served as senior director, Government Affairs & Policy, SSA at J&J.
[hearken id="daily-maverick/9303"]




 Potential Early Harvest AfCFTA Benefits in the Vaccine Value Chain, (p.68) The Futures Report 2021, Which value chains for a made in Africa revolution? UNDP, AfCFTA Secretariat
Disclaimer - University of Cape Town This email is subject to UCT policies and email disclaimer published on our website at http://www.uct.ac.za/main/email-disclaimer or obtainable from +27 21 650 9111. If this email is not related to the business of UCT, it is sent by the sender in an individual capacity. Please report security incidents or abuse via https://csirt.uct.ac.za/page/report-an-incident.php.
Potential Early Harvest AfCFTA Benefits in the Vaccine Value Chain, (p.68) The Futures Report 2021, Which value chains for a made in Africa revolution? UNDP, AfCFTA Secretariat
Disclaimer - University of Cape Town This email is subject to UCT policies and email disclaimer published on our website at http://www.uct.ac.za/main/email-disclaimer or obtainable from +27 21 650 9111. If this email is not related to the business of UCT, it is sent by the sender in an individual capacity. Please report security incidents or abuse via https://csirt.uct.ac.za/page/report-an-incident.php.