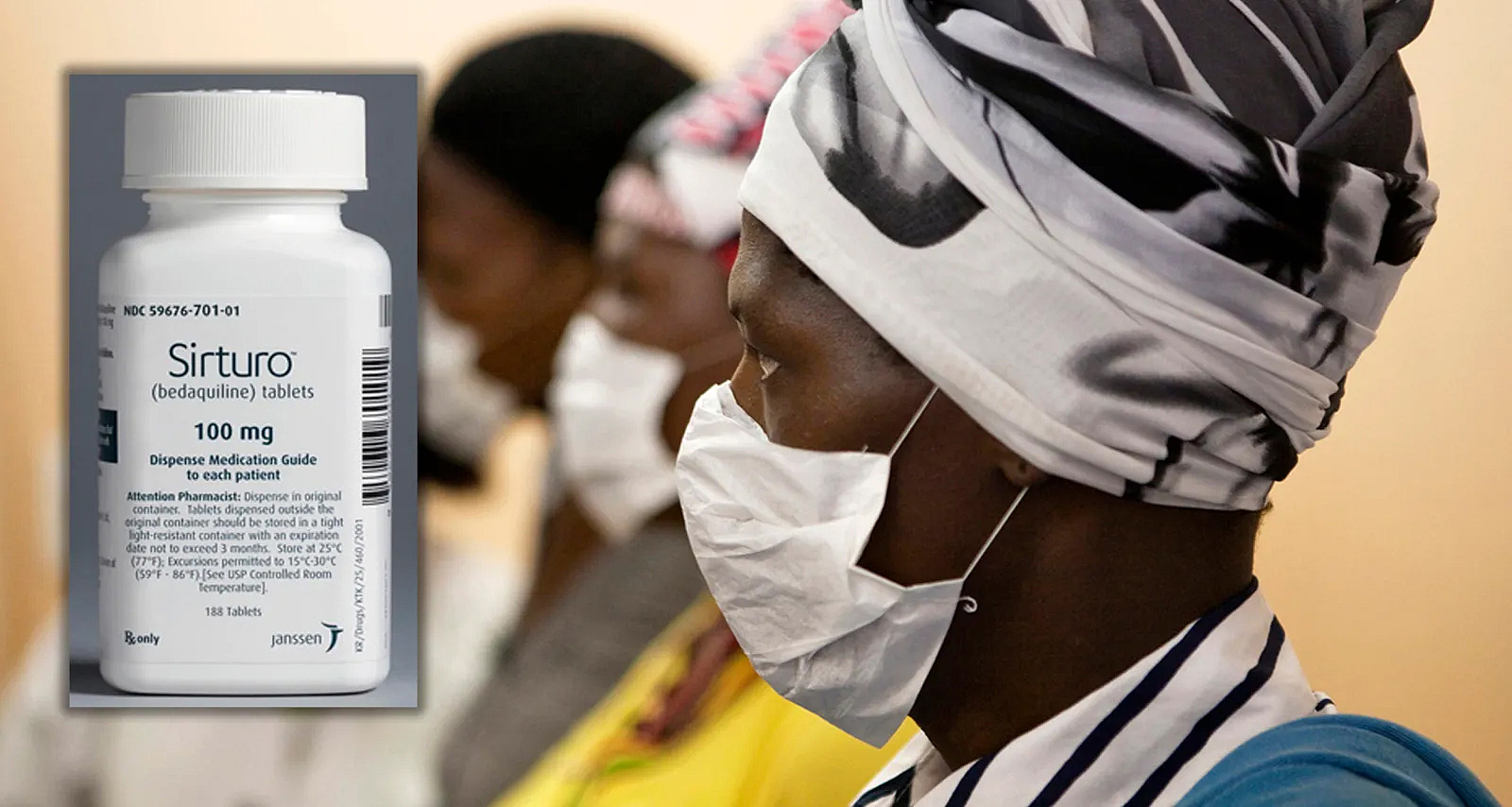
In an unprecedented move, South Africa’s Competition Commission will investigate the American drugmaker Johnson & Johnson (J&J) for the high price it has been charging the country for the tuberculosis (TB) medicine bedaquiline, as well as for extending the tablets’ 20-year patent until 2027 to block cheaper generics from entering the country.
South Africa has one of the highest TB disease rates in the world, and, despite it being curable, TB is the country’s top killer.
Bedaquiline is used to treat multidrug resistant (MDR) TB, a type of TB for which standard treatment doesn’t work. The drug, which is mostly used in combination with other medicines, has to be taken for six months and is considered a game changer because it has replaced treatment – up to two years of long, painful injections with serious side-effects such as hearing loss – with lower cure and higher death rates.
The Health Department started to give bedaquiline to everyone who needed it in the public health sector in 2018.
The commission’s investigation was made public on Thursday by the department and the legal organisation, the Health Justice Initiative (HJI), at a media briefing by Doctors Without Borders (MSF). The commission’s media spokesperson, Siyabulela Makunga, has confirmed to Bhekisisa that the probe is under way.
Read more in Daily Maverick: We probably all know someone who has or has had tuberculosis — we just don’t know it
The commission investigates matters when it has reasonable suspicion that there was exploitative or unethical behaviour.
The latest announcement follows shortly on the heels of HJI’s exposure last week of J&J’s “take it or leave it” bullying tactics in its secret Covid vaccine contract with the government (the Pretoria High Court ordered the Health Department to release the documents). The agreement revealed that J&J charged South Africa 15% per dose more than what it sold shots to the European Union for and 25% more than the estimated not-for-profit price.
How does – and will – South Africa pay for bedaquiline?
As things stand, from 1 October, South Africa will be paying more than double the price for bedaquiline compared with many other countries buying the drug.
Currently, the Health Department buys a six-month course per patient for R5,400. But from 1 October, when a new contract with J&J starts, South Africa will pay R5,500 for a course, while countries that procure bedaquiline through the Stop TB partnership’s Global Drug Facility will be able to buy it for R2,446 ($130).
The Global Drug Facility uses pooled procurement to negotiate payments, which makes it possible to bargain for lower prices for TB medicines because the drugs can be ordered in large quantities. But countries such as South Africa, which have open tender systems, can’t legally buy medicine through such systems, which is why the Health Department buys bedaquiline directly from J&J (Janssen).
The Competition Commission believes J&J could be in contravention of Section 8 of the Competition Act, which deals with excessive pricing and exclusionary conduct.
South Africa’s October agreement with J&J has a clause which allows for prices to be renegotiated, but that has not yet happened.
“We are enraged to witness that J&J prioritises profit over the needs of the most vulnerable populations in a country with a high burden of drug-resistant TB,” says Candice Sehoma, MSF’s Access Campaign advocacy officer. “We call on J&J to offer the same price of R2,446 for bedaquiline to the South African government as they have offered to countries that are part of the Global Drug Facility deal.”
Why does J&J still have a patent for bedaquiline in South Africa?
The patent for bedaquiline compounds in South Africa expired in July, but was extended to 2027 through a practice activists refer to as evergreening, whereby pharmaceutical manufacturers make small, trivial changes to medicines or their use in order to keep their monopoly in the market.
Extended patents are called secondary patents and original patents primary patents.
Evergreening, which South Africa’s patent laws allow because secondary patent applications are often not scrutinised sufficiently, makes it possible for drugmakers to keep patents far beyond the standard 20 years and so prevent competitors who make the same products for cheaper from entering the market.
“The Competition Commission believes J&J could be in contravention of Section 8 of the Competition Act, which deals with excessive pricing and exclusionary conduct, which, in this case, refers to the practice of evergreening [because it results in excluding others from the market],” says HJI’s head, Fatima Hassan. “We believe this is unprecedented. We do not know of other investigations by the Competition Commission into a pharmaceutical company for evergreening.”
What South Africa can learn from India – and why our patent laws need to change
Some countries, such as India, have found ways to prevent the granting of unjustified secondary patents by doing strict reviews of patent applications which examine the novelty and inventiveness of a medicine that a pharmaceutical company would like to extend the patent for. Using this system, India’s patent office denied J&J’s application for a secondary patent for bedaquiline in March. Had the patent been granted, J&J would have enjoyed a monopoly for four more years and it would have been illegal to sell cheaper generic versions of the drugs.
Because of this ruling, India can now make and sell generic bedaquiline (after the branded product’s patent expired in July), but the country is not allowed to export these products to countries such as South Africa, where secondary patents for bedaquiline have been granted.
Something for our legislators to consider is why we haven’t fixed our [patent] laws, to ensure we don’t expose ourselves to these kinds of exploitative practices.
The Global Drug Facility agreement makes it possible for some countries, where J&J holds secondary patents for bedaquiline, to buy a specific generic product via the facility, but because South Africa isn’t part of that agreement, the country is unable to do so.
Read more in Daily Maverick: New drug-resistant TB medicines will save lives but high costs remain a concern for health equity
South Africa’s patent office falls under the jurisdiction of the Department of Trade, Industry and Competition and its conduct has been contentious. For the past decade, health activists have been advocating for the legislation to be changed so that evergreening is controlled.
Russell Rensburg, the director of the Rural Health Advocacy Project at Wits University, explains:
“Something for our legislators to consider is why we haven’t fixed our [patent] laws, to ensure we don’t expose ourselves to these kinds of exploitative practices. Fixing the patent laws is essential to addressing health inequity.”
On 22 September, world leaders will gather at the United Nations General Assembly in New York for a high-level discussion on the progress and stumbling blocks in the fight against TB – including practices such as evergreening.
J&J, which the Competition Commission has informed about its investigation, responded as follows to Bhekisisa’s request for comment: “Johnson & Johnson is a longstanding and committed partner in South Africa’s fight against multidrug-resistant tuberculosis. Today, all patients in South Africa who require bedaquiline, our medicine for multidrug-resistant tuberculosis, have access to it thanks to our collaboration with the government of South Africa and other partners, which has contributed to a steady decline in TB incidence. We will continue to work collaboratively with our partners to ensure we can achieve our shared goal of ending TB.” DM
This story was produced by the Bhekisisa Centre for Health Journalism. Sign up for the newsletter.