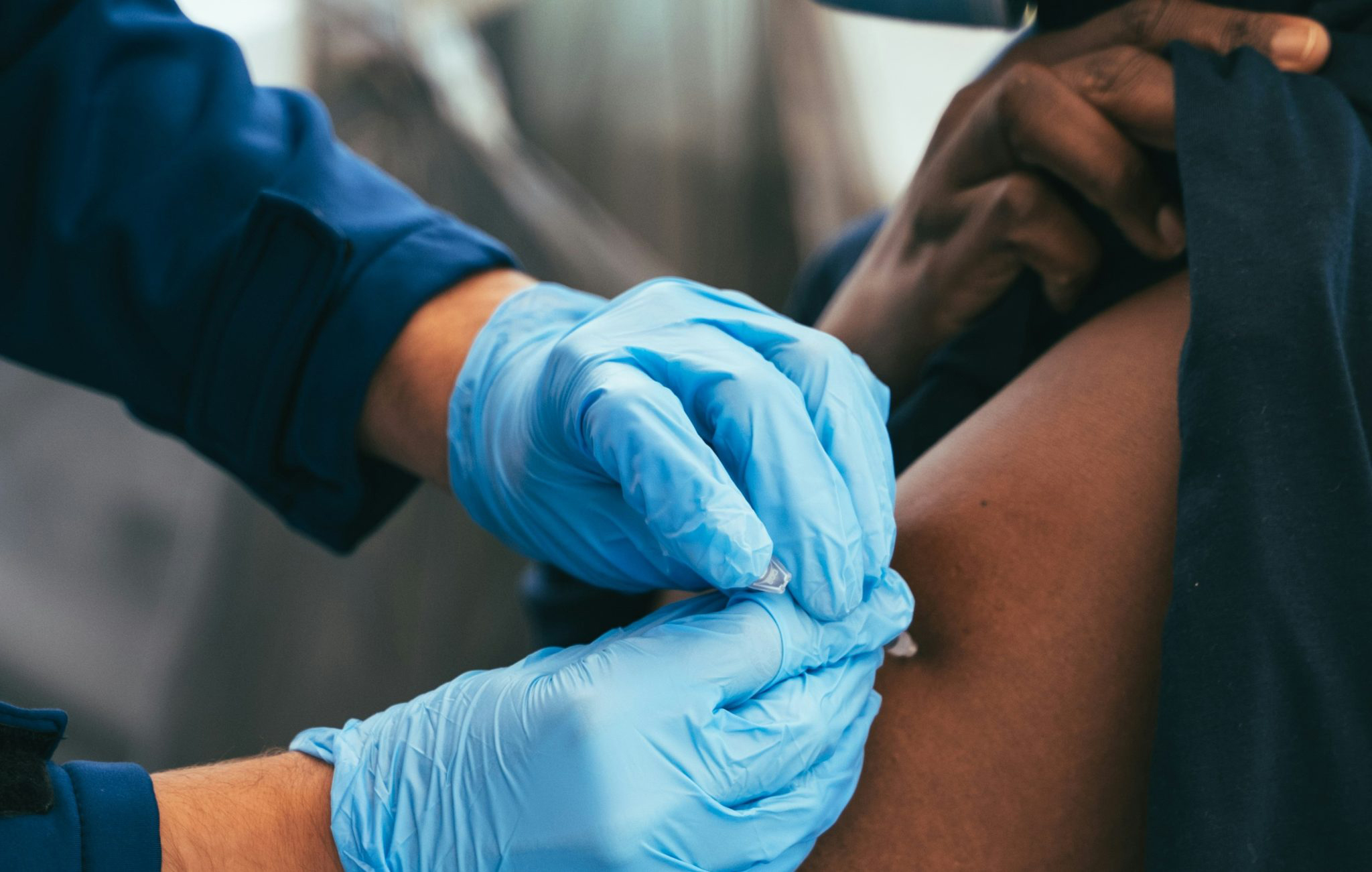
The first jabs in a much-anticipated clinical trial of an experimental tuberculosis (TB) vaccine have been administered at a clinical trial site at the University of the Witwatersrand in Johannesburg.
Up to 20,000 people are anticipated to take part in the study, according to study sponsor, the Bill and Melinda Gates Medical Research Institute (Gates MRI). The study will be conducted at 60 different sites in South Africa, Zambia, Malawi, Mozambique, Kenya, Indonesia and Vietnam. The researchers estimate that between 50% and 60% of the study participants will be in South Africa.
The experimental vaccine called M72/AS01E (M72) made waves in 2018 and 2019 when it was found to be around 50% effective at preventing people with latent TB infection from falling ill with TB over a three-year period in a phase 2b clinical trial.
In June 2023, it was announced that, after some delays, $550-million in funding had been secured for a phase 3 study of the vaccine. Medicines or vaccines are typically registered and brought to market only after being shown to be safe and effective in large, phase 3 clinical trials.
While most cases of TB can be cured using a combination of four antibiotics for four or six months, TB rates are declining relatively slowly and it is widely thought that an effective vaccine would help bring rates down much more quickly.
The World Health Organization estimates that at the level of protection seen in the phase 2b trial, the vaccine could potentially save 8.5 million lives and prevent 76 million people from falling ill with TB over a 25-year period. The one TB vaccine we already have, called Bacille Calmette-Guerin (BCG), is more than a century old and provides only limited protection against severe illness for children and no protection for adolescents or adults.
“Reaching phase 3 with an urgently needed TB vaccine candidate is an important moment for South Africans because it demonstrates that there is a strong local and global commitment to fight a disease that remains distressingly common in our communities,” said Dr Lee Fairlie, national principal investigator for the trial in South Africa, in a media statement released by Gates MRI.
“South Africa also has considerable experience with TB- and vaccine-related clinical trials and a strong track record for protecting patient safety and generating high-quality data essential for regulatory approvals.” Fairlie is also the Director of Maternal and Child Health at the Wits Reproductive Health and HIV Institute at Wits University.
The initial response from TB activists was positive.
“TB Proof (a South African TB advocacy group) is delighted that the M72 phase 3 trial has been launched,” the organisation’s Ruvandhi Nathavitharana and Ingrid Schoeman told Spotlight. “Having an effective TB vaccine is critical for TB elimination efforts.”
While he said it was good to finally see the phase 3 trial of M72 get under way, Mike Frick, TB co-director at Treatment Action Group, a New York-based TB advocacy organisation, continued: “The fact that we had to wait so long between phase II and phase III says everything one needs to know about the headwinds – financial, political, commercial – that TB research is up against.”
How the study will work
Half of the up to 20,000 study participants will receive the M72 jab and the other half a placebo. The vaccine is administered as two intramuscular injections given a month apart. After being jabbed, study participants, all aged 15 to 44, will be followed for four years from the date of the first study participant being enrolled to see if they fall ill with TB.
“The plan is to complete enrolment in two years,” Fairlie and Alemnew Dagnew, clinical lead for the trial, told Spotlight in response to written questions. They explained that the actual duration of the trial would depend on how long it took for 110 people in the study to fall ill with TB. The Gates MRI statement notes the study is expected to take around five years to complete.
According to Fairlie and Dagnew, the majority of study participants (around 18,000 people) will be people who are HIV negative and who have latent TB infection – that is to say, people who have TB bacteria in their lungs, but who are not ill with TB. Latent TB infection is thought to be very common in South Africa and only around 10% of people with latent infection ever fall ill with TB. In the study, latent infection will be tested for using a type of test called an IGRA (Interferon-Gamma Release Assay).
Around 1,000 HIV negative people with no TB infection will also be recruited to the study. This is being done to make sure the vaccine is safe and effective in this group of people – while latent infection will be tested for in the study, in the real world such testing may not always be feasible prior to vaccination.
It is anticipated that 1,000 of the 20,000 study participants will be people living with HIV. Establishing how well the vaccine works in people living with HIV is important since around 13% of people in South Africa are living with HIV and HIV substantially increases the risk of falling ill with TB.
The main phase 2b study of M72 did not include people living with HIV although another phase 2 study looked specifically at the safety and immunogenicity of M72 in people living with HIV – according to Fairlie and Dagnew, “that trial “was completed and supported the inclusion of such participants in a phase 3 trial”.
Smaller than previously thought
When funding for the phase 3 trial was announced last year, it was estimated that 26,000 people would participate in the study. That number has now been revised down to 20,000.
“As a result of ongoing discussions between the institute and our funders, the decision was taken to review the study protocol with the intent of simplifying the study given its size and complexity.
“This will not affect the safety of the trial. It is common to continue to refine a protocol. We found a way to expedite the study that would potentially allow us to offer the public health impact of this vaccine to those in need sooner. All partners, including the trial funders, are fully aligned to the protocol refinements,” Fairlie and Dagnew explained to Spotlight.
“Some assumptions used to inform the design of the first protocol were deemed overly conservative, so the clinical team used slightly less conservative assumptions on vaccine efficacy and TB incidence rate, thus allowing for a reduction in the number of participants in the trial, while still retaining the primary goal of confirming the safety and efficacy of the M72/AS01-E-4 vaccine for prevention of TB, guided by the final results of the phase 2b study completed several years ago.”
Planning for access
The development of M72 has taken a somewhat unusual path – with the pharmaceutical company GSK leading development up to the end of phase 2b and then largely passing the baton to Gates MRI with the conclusion of a licensing deal in 2020.
GSK has come in for some criticism for not moving more quickly after the initial publication of the phase 2b results in 2018. A ProPublica article published last year suggested that the development of M72 slowed because GSK was focusing on more profitable vaccines.
According to the Gates MRI statement, GSK continues to provide technical assistance to the Gates MRI, supplies the adjuvant component of the vaccine for the phase 3 trial, and will provide the adjuvant post licensure should the trial be successful. An adjuvant is an agent included in the vaccine that improves the immune response elicited by the vaccine – in the case of M72/AS01E the AS01E refers to the adjuvant made by GSK.
This ongoing dependence on a single company for the adjuvant has some activists worried.
“We are concerned about reports that scaling this vaccine may be difficult due to limited availability of the vaccine adjuvant. Access for everyone who needs it should be part of the early phases of the research process – not an afterthought,” said Nathavitharana and Schoeman.
“The press release announcing the study’s start in several places refers to the ‘complexity’ of ‘developing and ensuring access’ to a new vaccine. Part of the unspoken complexity here is the opaque licensing deal GSK and Gates MRI signed in 2020 in which GSK gave rights to develop and commercialise M72 to Gates MRI while retaining control over the AS01E adjuvant,” Frick told Spotlight.
“There are legitimate concerns that the fine print of this arrangement could work against equitable access, but terms of the license remain unknown to the public.”
When asked about supply concerns, Gates MRI told Spotlight: “Gates MRI collaboration with GSK includes provisions to ensure there is sufficient supply of adjuvant for the clinical development and first adoption in low-income countries with high TB burden, at an affordable price, should the vaccine candidate be successful in phase 3 trials and approved for use. For broader implementation, GSK has committed to working with its partners to ensure there is sufficient supply.” DM
This article was published by Spotlight – health journalism in the public interest. Sign up to the Spotlight newsletter.